Lepisma saccharina (Linnaeus, 1758)
Also known as: (Common) silverfish, ‘Bookworm’, FishmothTable of Contents
Figure 1a (Left): This group of wingless insects is often introduced as a voracious reader which savours every word it digests, in a literal sense of the word. However, the exact species identity of this specimen as the common silverfish, Lepisma saccharina, will be challenged in this article, since it has excessive hair (chetae) on its head. Photo credit: Alessandra e Rocco Marciano (Permission pending). Original source indicating it to be Lepisma saccharina. Figure 1b (Right): An actual confirmed Lepisma saccharina specimen. Image searches often turn up with insects possessing both morphological traits (refer to Figure 10a for another example). Photo credit: Christian Fischer, licensed under Creative Commons.
Media 1: Are Silverfish dangerous? Spoiler: The answer is No, though the damage they are capable of in large numbers can be potentially annoying at best and horrific at worse. Again, note the silverfish featured might not be the species Lepisma saccharina. Credit: user jackdoggerdog. Obtained from YouTube under fair use.
Why isn't it a Fish? Why is it a Bookworm?
Despite being called a ‘Silverfish’, it is not to be confused with an actual fish, such as the similarly named Antarctic Silverfish (to be more precise, a silver-colored member of the paraphyletic group of underwater organisms that generally lives underwater). This name is likely a reference to its silvery-grey scales, as well as their fish-like movement while running across the floor at high speeds (Borror et al., 1981).
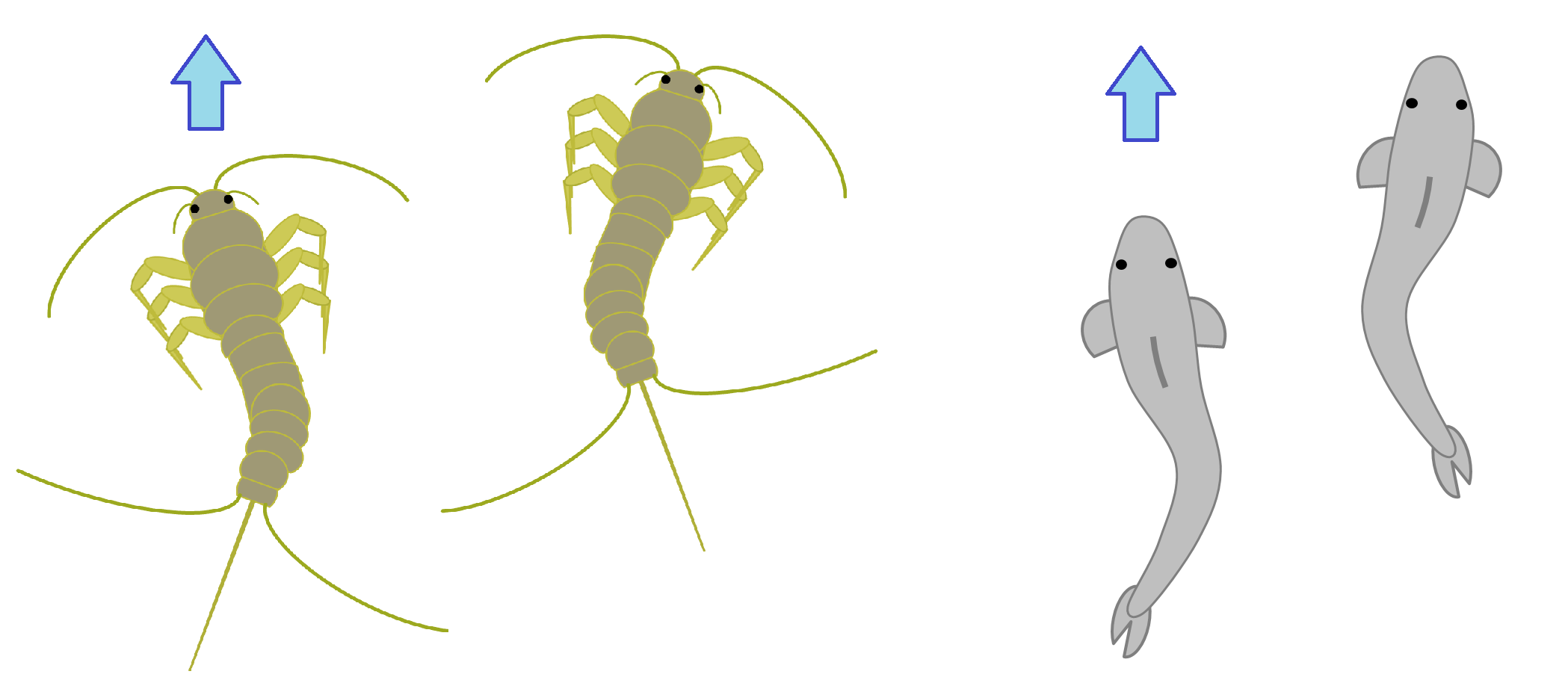
Figure 2: Silverfish (Left), when sprinting, tend to move in a wave like fashion, similar to actual fish (Right). This similarity, coupled with their silvery scales, is the most likely reason for their common name.
The silverfish is one common insect referred to as a ‘bookworm’, since it consumes paper (Gallo, 1961); However, this name is shared with booklice (Koehler, 2016), or a Psocoptera insect larvae (That again has nothing to do with true, parasitic lice), and other common insects that also eat paper ranging from larvae of beetles to cockroaches and termites (Gallo, 1961). Similarly, all of them have nothing to do with actual 'worms' (e.g. Earthworms, Flatworms, Roundworms and any other worms that are not insects), which is a term colloquially used to describe any small and elongated creature in general.

Figure 3a (Left): Mild silverfish damage on paper. More horrific images are available on Google, especially for older paper; Do not search if you have Trypophobia (i.e. warning: gross). Photo credit: Patricia Alder and Michael Waldvogel, NC State University, Department of Entomology, College of Agriculture and Life Sciences. Figure 3b (Right): A booklice, Trogium pulsatorium, another common pest responsible for damaging paper but isn't related to Silverfishes. Photo credit: Tony Willis, licensed under Creative Commons. Not shown: beetle larvae, cockroaches and termites due to their sheer diversity.
Morphology and Identification
To identify silverfish in general, look out for a flightless, dorsal-ventrally flattened insect with prominent antennae, two long cerci and one long caudal/median filament (Delany, 1954; Grimaldi & Engel, 2005). For assistance, this section will first distinguish between silverfish and bristletails, the other major group of flightless insects, before going into detail of its morphology and comparison with two other commonly mistaken species, the European Silverfish or Firebrat, Thermobia domestica, and the Giant Grey Silverfish or Long-tailed Silverfish, Ctenolepisma longicaudata.
A glossary for the names of parts in general can be accessed here for clarification purposes.
Comparisons with Bristletails
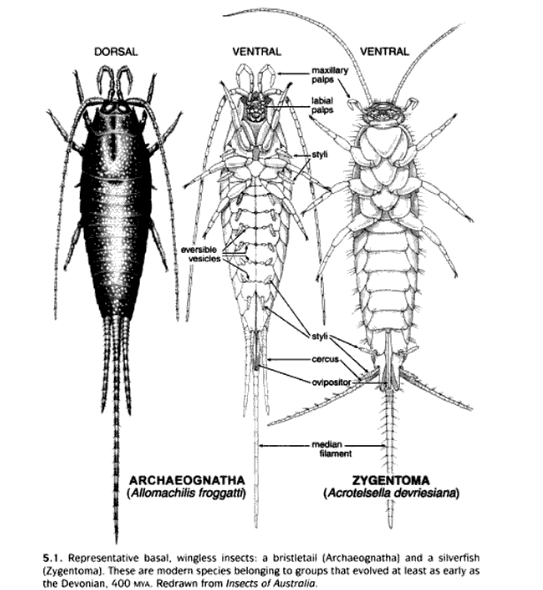
Figure 4: A related bristletail (left) compared to a silverfish in general (right). Both are living representative of flightless insects, but diverged much earlier in their ancestry (More information under Phylogeny). Diagram from Grimaldi & Engel, 2005.


Figure 5a (Left): An unidentified bristletail from Family Machilidae with a stout thorax segment. Photo credit: Flickr, by Nicky Bay, licensed under Creative Commons.
Figure 5b (Right): Side view of a specimen of Lepisma Saccharina, which is dorsal-ventrally compressed throughout. Photo credit: Blue Sky Pests.
Silverfishes were once grouped in the same Order as Bristletails under Thysanura (more information under Taxonomy). Nevertheless, key morphological cues such as being more dorsal-ventrally compressed, lack of eversible vesicles and lack of styli at the thorax section, combined with phylogenetics data have favored taxonomic revision (Mendes, 2002, Grimaldi & Engel, 2005, Misof et al., 2014), as well as acting as cues for distinguishing between members of both groups.
Adult
The following specimen (Figure 5a, 5b) was collected in Singapore in an urban environment, Block S1A, NUS. It was identified as Lepisma saccharina mainly by its colour, size, labial palps and relative lack of hair (chetae) near the head area. Its caudal/median filament was damaged during capture and preservation. It was preserved in 70% Industrial grade ethanol.

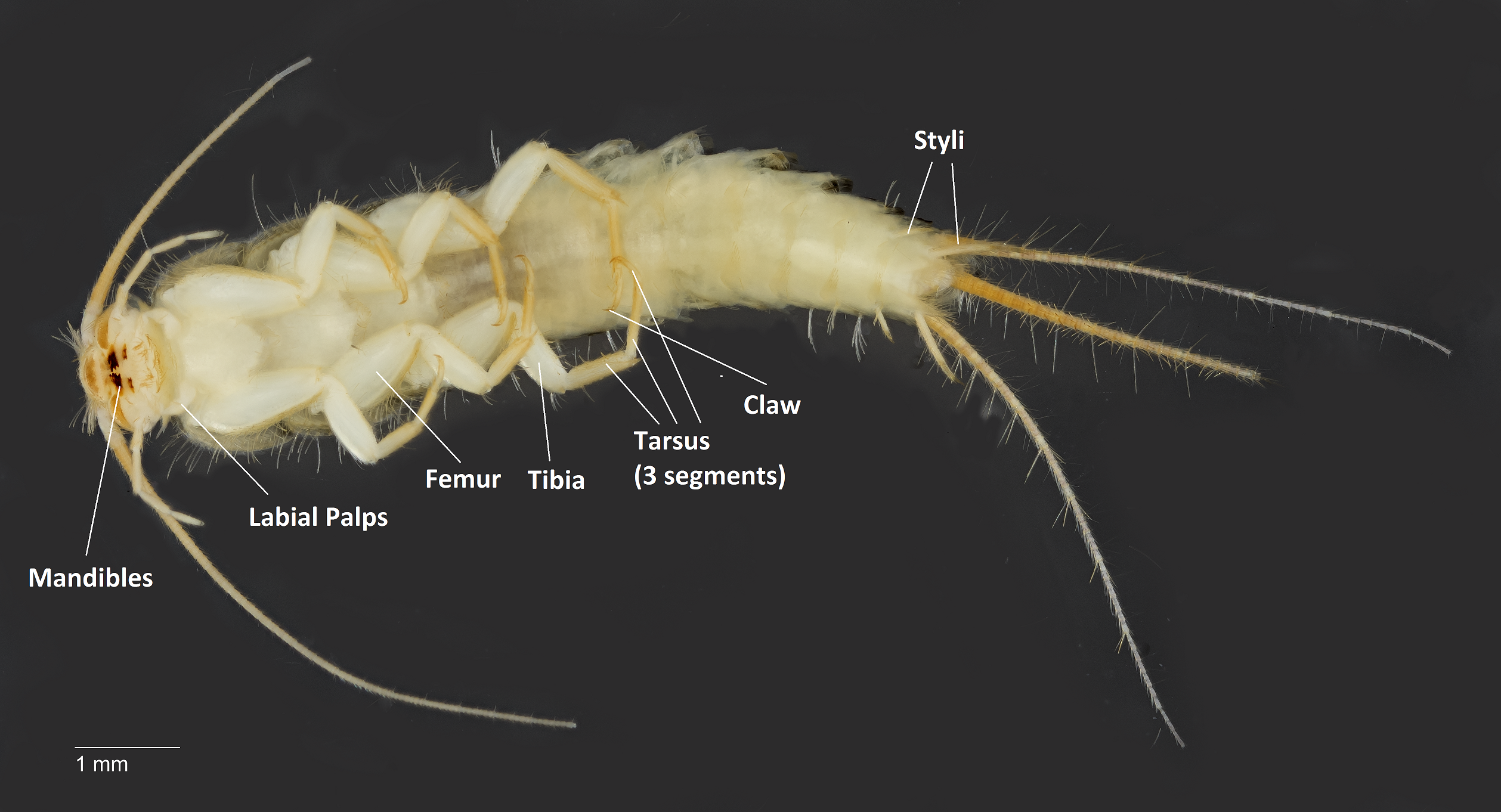
Figure 6a (Dorsal view) & 6b (Ventral view): labelled silverfish for reference on jargon pertaining to insect parts used in the description. General anatomical information was derived from the works of Delany, 1954. Specimen is likely to be a late nymph or adult. Photo credit: Kristy and Arina (Her page on the Oriental Fruit Fly is here).
Size: 8mm to 15mm (Based on estimates from current specimen collected & Klass, 2009), head to abdomen; upper limit of >20mm (based on eyewitness account, though suspected to be a related species, Ctenolepisma longicaudata). Dorsal-ventrally flattened.
Color: Black Eyes and mandibles. Uniformly slate grey, reflective upper dorsum surface. Yellow or brown-yellow appendages (antennae, limbs, cerci, filament), as well as yellowish chitinous yellow hair (or chetae) and ventral surface.

Figure 7: Close-up showing the black compound eyes of the silverfish (center of image), golden-yellow chetae and its grey coloured scales in great detail. Photo credit: Flickr, by Laurie Knight, licensed under Creative Commons.
Head: Mandibles are darker in coloration, and forms a simple mandibulate mouthpart. Has a pair of long, segmented sectaceous antennae. Simple compound eyes. Maxillary palps are long with prominent segments. Labial palps are short and broad, especially the apical segment. Has some chetae, though not well-documented.
Thorax: Covered in gray scales with yellow chetae. Three distinct segments giving rise to legs.
Abdomen: Up to eleven segments for fully molted adults. Two pair of styli on second last and last tergum. Tergum is truncate or blunt. Has two long cerci and a long caudal or median filament (occasionally misreported as a third cerci) giving an appearance of three tails.
Genitalia: Females have a long ovipositor below the caudal/median filament (Refer to section on Reproduction).
Legs: Robust and fleshy, especially the femur and tibia. Tarsus has three segments, ending in a single hooked tarsal claw.
Summary of key morphological traits:
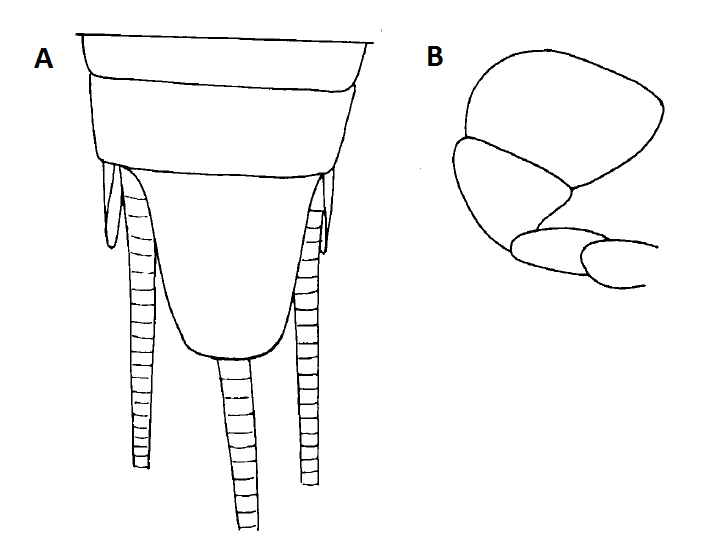

Figure 8a (Left): Two simple, crucial morphological cues that identifies Lepisma saccharina from other closely related silverfish in the same family/ genus; A: Blunt/truncate apex of last tergum (last section of abdomen); B: Broad apical segment of labial palp. Diagram from Wygodzinsky, 1972. Figure 8b: cropped images from my specimen for side-by-side comparison, which resulted in my deduction that the specimen is Lepisma saccharina.
All of the above description was compiled from personal observation, descriptions of related species and help from expert identification (Delany, 1954; Wygodzinsky, 1972; Grimaldi & Engel, 2005). However, I still have doubts over my species identification as its cerci are longer than expected (refer to Figure 1b), and as a result it might still be a different species not listed in this page. I would highly appreciate cross checking from an expert on this matter.
Comparisons with Thermobia domestica

Figure 9: While similar in size, the Firebrat, Thermobia domestica looks very different from the common silverfish. Photo Credit: Flickr, by Malugo, licensed under Creative Commons.
Thermobia domestica, also known as the European silverfish or Firebrat, is about the same size as Lepisma saccharina. Nevertheless, its orange-yellow coloration with black stripes and grey molts is a dead givaway for distinguishing it from a common silverfish by appearance alone (Klass, 2009). In fact, its common name, Firebrat is derived because it stays in areas with high temperatures, such as behind ovens and electronics, since it prefers higher temperatures for its eggs to hatch and grow (DeVries & Appel, 2013).
Comparisons with Ctenolepisma longicaudata


Figure 10a: An image of Ctenolepisma longicaudata. Notice its abundant hair-like structures, as seen in the silverfish in Figure 1a. and Media 1. Half of all image searches for Lepisma saccharina actually show a similar creature, which was extremely worrying as this species might have been confused as another 'common silverfish' all along, especially for smaller specimens. Photo credit: Pudding4brains, under Wikimedia for use in Public Domain. Figure 10b: close-up of the head, ventral view. Notice its labial palps are different from Lepisma saccharina's. Photo credit: Pests and Diseases Image Library. Image Source.
Warning: This species is DIFFICULT TO TELL APART from Lepisma saccharina!
Sources have conflicting opinions over whether a specimen is Ctenolepisma longicaudata or Lepisma saccharina; many images searches with either scientific name still returned with images of either species, though more prominent in Lepisma saccharina's case. To make matters worse, its diet, distribution and status as a pest is almost identical to the common silverfish (Lindsay, 1940). In short, the following diagnosis segment is based entirely on my judgement and some cues picked up from Wygodzynsky, 1972, Houseman, 2016 and by piecing together information from forums by insect enthusiasts on this matter:
- It is generally agreed that an adult silverfish that reaches about 20mm in size is Ctenolepisma longicaudata.
- The presence of thick chetae around the head portion, as well as extremely long cerci and caudal/median filaments (almost equal or longer than its body length) seem to be a discriminating factor between it and Lepisma saccharina, but it is not officially stated. It is still unknown if the presence of chetae and cerci length is a result of intraspecific variation or indeed a result of two differing species.
- Wygodzynsky, 1972 indicated that besides similar coloration, it also has a blunt/ truncate apex of the last tergum, and thus it is easy to confuse the two species without size comparisons. However, its labial palps are shaped very differently (Refer to Figure 10b); the apical segment is broad but more oval in shape, the segment before is less triangular, and the connecting segments to the head is more robust compared to Lepisma saccharina's (Compare with figure 8b).
- For pest management purposes, this isn't an issue as similar tactics can be used for either species, other than that Ctenolepisma longicaudata is more tolerant of less humid conditions while being more vulnerable to chemical control. However, this might have implications for research purposes.
Verification with an expert is required.
Summary Table for Silverfish identification:
| Traits |
Lepisma saccharina |
Thermobia domestica |
Ctenolepsima longicaudata |
| Color |
Slate grey |
Yellow, grey molts and black stripes |
Slate grey |
| Maximum Size |
15mm |
15mm |
20mm |
| Chetae on head? |
Few (from confirmed specimens) |
Plenty |
Plenty |
| Preferred breeding conditions |
Moderate temperatures, high humidity |
High temperatures, moderate humidity |
Moderate temperatures and humidity |
| Most reliable identification feature |
Unique labial palps |
Unique coloration, preference for warmer environments |
Large size, long cerci/ caudal filament |
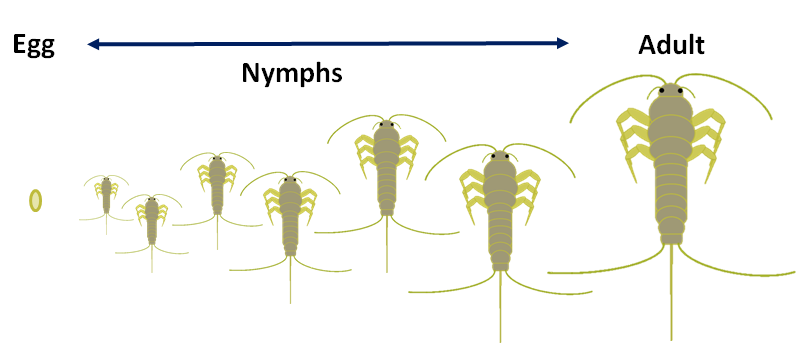
Nymph

Figure 11: Newly hatched silverfish nymph. Notice the lack of scales and its small size (~3mm, no scale bar on original image) but otherwise it strongly resembles an adult. Original source: Jacksonville, Duval County, Florida, USA, January 31, 2009. Size of the nymph is about 3mm. Photo credit: user goingcarcrazy in 2009 as a query on bugguide.net.
Due to its small size and lack of scales, it is difficult to confirm the exact identity of nymphs, though the above image is suspected to be a nymph of Lepisma saccharina. The nymph is described in literature to be similar to the adult (as a result of incomplete metamorphosis; refer to Figure 13a below) except it lacks the characteristic slate grey color (Morita, 1926), presumably as it has yet to develop scales until its third molt (Resh & Cardé, 2003).
Reproduction
Sturm, 1956 also detailed Silverfish courtship behavior, reproduction and copulation in detail. As the original text was in German, I’ve included only some relevant images that hopefully speaks a thousand words, and based my findings on a summary in English + Google translate.
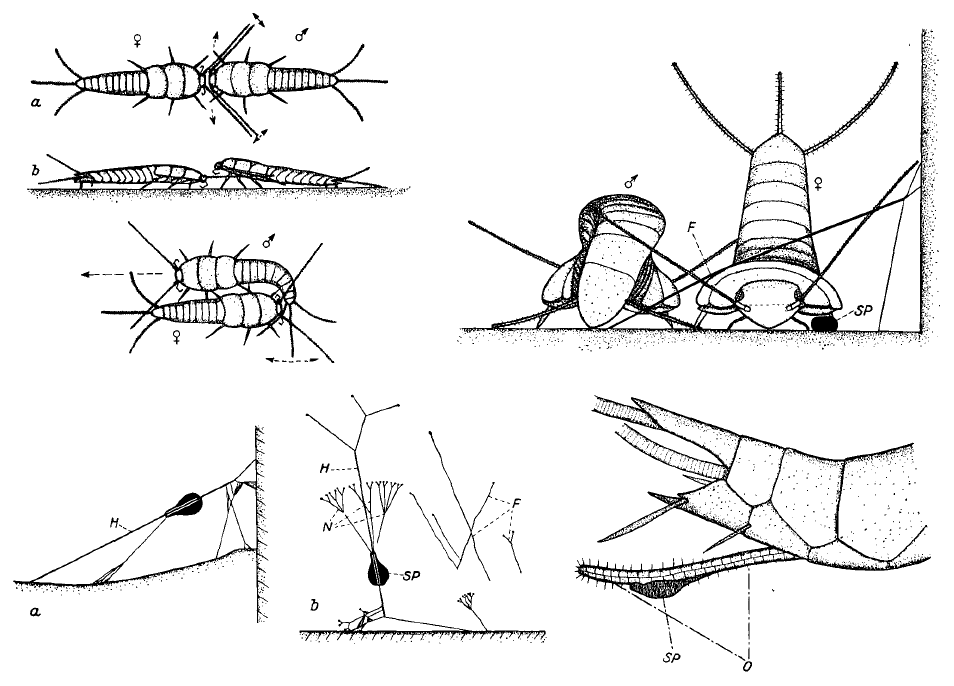
Figure 12: Top Left: Courtship display of Silverfish involves complex dances. Top Right: The male first deposits a sperm capsule, or spermatophore (SP) on the ground before spinning silk, anchored to a surface such as a wall or object. Bottom left: The spermatophore is picked up by the female and suspended on silk. Bottom Right: The spermatophore is spread along the ovipositors of the female. Diagram is compiled from various images from Sturm, 1956.
Before silverfish reproduce, they carry out a ritualistic courtship dance involving three steps lasting over half an hour. The male first contacts his antennae with the female, repeatedly backing off and returning to this position (Top left, Figure 10, top). The male then runs away and the female chases him. After which, the male and female will be side by side, head-to-tail, with the male vibrating his tail against the female (Top left, Figure 10, bottom). Finally, the male lays a spermatophore (sperm capsule) on the ground after this elaborate preliminary display with the female (I need help clarifying if the male or female suspends the spermatophore later).
Several threads are spun along the ground, one end anchored to an elevated object such as a wall surface. When the female touch the lower threads with her feet, she raises her abdomen to contact the main thread and is lead to the vicinity of the spermatophore (Top right, then Bottom right, Figure 10). The Spermatophore will then be taken up by the ovipositors (Bottom right, Figure 10).
One additional interesting note on silverfish silk:
Composition of the silverfish’ silk threads was not analysed until recent years, and postulated to be similar as it was performed on a related species, the (Giant) Grey Silverfish Ctenolepisma longicaudata (Figure 10a, 10b). As silverfish do not produce threads on a regular basis, its structure is composed mostly of random coils (different from silkworm silk by lacking Beta pleated structures), as such being less ordered at the molecular level. As a result, silverfish silk is weaker in tensile strength, which is consistent with its temporary, short-term function during the breeding process (Walker et al., 2013).
Life Cycle
Females lay about 7-12 eggs at a time but may lay several batches over a period of weeks, year-round in secluded places, such as behind books or on closet shelves. Occasionally, they lay eggs in the open. The eggs are whitish, oval and about 0.8 mm. They hatch in two to eight weeks, depending on temperature (Koehler, 2016).
Young silverfish, on hatching, resembles their parents entirely, except that the first stage possesses an egg tooth and they lack scales until the third molt (Resh & Cardé, 2003). It undergoes a series of six to seven molts to reach an adult stage. The first three molts occur faster than subsequent ones. Despite the low number of eggs produced compared to most insects, more than 80 per cent of nymphs generally survive to adulthood, and thus they multiply relatively quickly (Morita, 1926). The rate of growth is highly dependent on temperature and humidity, as it Iimprove their metabolic rate and growth, though not as much as another related species, the Firebrat or Thermobia domestica (figure 9, above) (DeVries & Appel, 2013).
Silverfish can survive for up to three years (Resh & Cardé, 2003).
Figure 13a: The illustrated life cycle of silverfish can be relatively boring at first glance, since they undergo incomplete metamorphosis.
Figure 13b: A clipart I’ve made, constructed via basic shapes from scratch in PowerPoint and uploaded by popular request. Feel free to use this cartoon for non-commercial purposes. Note that this clipart represents a generic silverfish for illustration purposes.
Behavior and Traits
Silverfish generally shun light as far as possible, often hiding in dark corners. Being dorsal-ventrally flattened, they easily seek refuge in small cracks or fissures in homes (Resh & Cardé, 2003; Koehler, 2016). Personal observation on a live specimen and while capturing the specimen for preservation has shown and verified from online sources that:
- The silverfish is highly inactive during the day. As a result, studying its behavior is not easy to do.
- It ends up alarmed if its antennae or cerci contacts is touched by a moving object.
- At night, it only crawls actively if left in near-total darkness; it will 'freeze' when exposed to a burst of strong light (e.g. from a torch), and move away from light sources.
- It is unable to climb up smooth surfaces.
- It is an avid runner, especially while evading capture and it is extremely easy to damage its antennae, cerci or caudal filament while capturing one. Finally, its faeces resemble tiny, black pellets.
- It can survive falls from great heights (Refer to Hasenfuss, 2002 for more information).
- It is documented to be indifferent to loud noises, and verified to be the case.
Silverfish were documented to take two to four weeks to regenerate clipped antennae and filaments, which are inferred to be frequently lost given their fragility. Surprisingly, cannibalism was also documented for silverfish in captivity (Morita, 1926).
Distribution and Habitat
The common silverfish is cosmopolitan, spotted in every major continent except Antarctica (Morita, 1926), though the Encyclopaedia of Life (EoL) has an unreferenced claim that the silverfish is native to the Palearctic (North Africa, Europe and northern Asia) and was subsequently introduced to the rest of the world, likely by humans (EoL, 2016).
The silverfish is most often found on old bookshelves in libraries, storerooms behind magazines and newspapers, and anywhere with paper product in urban areas (Morita, 1926; Gallo, 1961, Koehler, 2016). It is also typically found in small cracks in pantries, kitchens and bathrooms, or any area with high moisture/humidity (Resh & Cardé, 2003; Klass, 2009). In addition, curtains, linens, carpets and old clothing can occasionally be attacked as well, especially those based on plant fibres such as cotton (Morita, 1926, Koehler, 2016). Silverfish today are mostly documented as an urban species, traveling through pipelines across homes to find a feeding spot in human settlements (Klass, 2009).
Little is known about the silverfish in the wild, though records and the Encyclopedia of Life (EoL) document it to be found under leaf litter, rocks, wood mulch, logs and deadwood (Schaller, 1968; EoL, 2016), likely a result of its nocturnal lifestyle (Morita, 1926; Klass, 2009).
In Singapore, it is occasionally sighted in homes, offices and school/public libraries, according to personal anecdotes.
Diet
The silverfish is notorious for eating away at paper, book covers, and even glue from books in library, thus its designation as a ‘bookworm’ (Gallo, 1961; Klass, 2009). In particular, it will eat anything containing lignocellulose, sugar or starch, as it is capable of digesting cellulose. In nature, silverfish feed on deadwood, other plant material and detritus, and thus is an important detritivore (scavenger) of plant material, but this results in it being a nuisance pest once it invades urban environments and start eating up other types of materials from dead plants.
Its diet in human habitats consists of the following non-exhaustive list (Resh & Cardé, 2003; Klass, 2009; Koehler, 2016), including:
- paper, especially books, newspaper and magazines
- cardboard boxes and other cardboard-based material
- flour, cereals in kitchens
- cotton and cloth clothing
- untreated wallpapers
Pest Management
The Common Silverfish are not listed on both the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and the International Union for Conservation of Nature and Natural Resources (IUCN) Red List; this is likely due to the reputation of the common silverfish as a prolific pest, and thus it is likely to be of Least Concern.
Silverfish nevertheless are harmless to humans in general (refer to Media 1), except for distress or panic if misidentified as a grey cockroach and inconvenience caused if it happens to feed on important archives/ materials. Klass, 2009 has provided an extremely comprehensive list of all common methods used to detect, prevent and stop a silverfish infestation in homes, tying in with information from literature and repeated in some form in other sources, summarized below:
Detection
- Look out for damaged paper material, cardboard, woolen cloth with holes, especially on bookshelves, storerooms and unused closets.
- Coat a card with a flour and water paste, let it dry, and place it in a suspected area. Leave it untouched for two weeks or so, then return to check for tiny scrape marks or ragged edges.
- A container with flour and/or cardboard can be used as both a lure and a trap for actual specimens if it has a smooth inner surface, as a silverfish is unable to climb out of it.
Media 2: A youtube video showcased how to construct a DYI silverfish trap. Inspired, I attempted to recreate the trap to obtain a life specimen for observation. Credit: user How to .. Garden, Garage and House Channel. Obtained from YouTube under fair use.
However, testing in Singapore revealed that the jar was inefficient and most silverfish are attracted to but not willing to end up in the trap. Since attempted captures were performed at the National University of Singapore (NUS), a combination of abundance of food supply and small local population/ sample size meant it was difficult to verify its effectiveness. Nevertheless, I would recommend using a more shallow structure, such as a Petri dish with a ramp or masking tape on the outer surface as an alternative set-up (see below) to improve your chances of capturing one.

Figure 14: Set-up of silverfish traps tried, using flour and cardboard scraps as bait. Left: the Flour jar. It was surrounded by black tape initially, but partially removed to show flour within. Silverfish were unable to climb into the jar or even detect it. Right: a petri dish with some flour and cardboard. I had a paper ramp for silverfish to climb in, but none took the bait. One silverfish was indeed attracted to the trap. However, it escaped after I failed to trap it under the cover. Photo: by Qi En.
Prevention
- Reduce moisture using a dehumidifier, and improve ventilation in bathrooms using fans that transfer moist air outside.
- Seal up cracks and crevices on walls, kitchen tables, etc. especially around pipes, windows, moldings, and cabinets; Caulking, plaster and various types of putty will suffice. This prevents accumulation of cellulose or starchy material, as well as deny silverfsih of hiding places.
- For small cracks and crevices which are difficult to access, one can use boric acid and diatomaceous earth (silicon powder), which are drying agents.
- Vacuum floors and carpets regularly to remove food debris.
- For Important private collections/ libraries, consider air-conditioning to reduce humidity, temperature and improve ventilation.
- Naphthalene bricks/ ‘mothballs’ on shelves will not only repel existing insects, but help prevent future infestation (Sahoo, 1990).
(Just for pest management practice purposes, consider its other common name, 'fishmoth', as a portmanteau of silverfish and moth(balls). This is a good way to remember that the latter repels not only moth larvae, but silverfish in general.)
Management
- Conduct a thorough inspection (call a professional if necessary), especially for rarely disturbed places such as storerooms, closets, and bookshelves to locate hiding places.
- Sticky cockroach traps can help in catching insects.
- As a last resort, chemical insecticides may be used, but do not use in food-storage cabinets or anywhere food is prepared (e.g. kitchen tables).
- In recent years, natural oil from the leaves of the Japenese Cedar (Cryptomeria japonica) was found to be effective as both a biodegradable repellent and insecticide against the silverfish, and could be employed over chemical insecticides (Wang et al., 2006).
Last but not least, if you’re good at catching insects or you’ve managed to trap one intact and alive in a jar, feel free to donate it to students taking Entomology classes. In NUS, this occurs during Semester 1 (August – October). If the silverfish specimen is sufficiently large (>1.5cm), it might be the Grey silverfish; If it doesn't resemble any of the above common species, you might even have a rare specimen!
Other Contributions in Scientific Literature
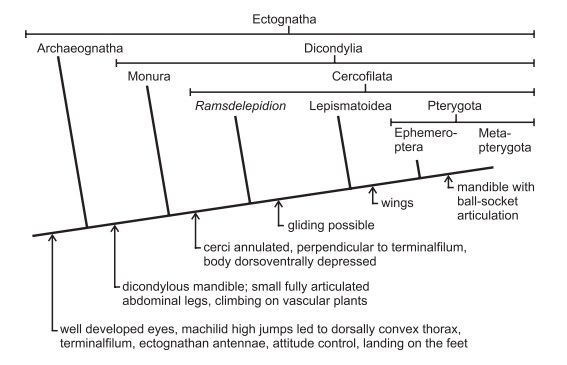
Figure 15: The above Cladogram is for illustration purposes of ancestral traits in various insect taxa, from flightless to flying ones. Silverfish belong in Lepismatoidea. This diagram is provided for reference purposes for those interested to pursue further reading. Diagram from Hassnfuss, 2008.
Silverfish are used as a model for developmental biology due to their simplicity and lack of a metamorphic stage (Mittmann & Scholtz, 2001). At the same time, they serve as evolutionary model for development of flight in insects; this was suggested because they possess gliding behavior while falling from a height (Hasenfuss, 2002) and possess structures with morphological similarities (Hassnfuss, 2008) to wings of modern insects from a common ancestor, but nonetheless didn’t develop into wings in the silverfish’s lineage.
Their midgut is of interest due to the ability to digest cellulose, and studies have used it as a model for epithelium formation in insects (Rost-Roszkowska et al., 2007). Being able to digest lignocellulose, they are currently being investigated alongside other insects (e.g. termites) for use in biofuel production from wood (Sun & Scharf, 2010).
Silverfish (Zygentoma) are not a high priority group for DNA sequencing studies compared to Diptera (Flies) and Lepidopteras (Butterflies/ moths). This is due to their larger genome size, low human impact and lower estimates for overall species count, resulting in lower priority for genome sequencing purposes (Evans & Gundersen-Rindal, 2003).
Taxonomy and Systematics
Type Information and Etymology
It is unclear if Linnaeus’ original type specimen for Lepisma saccharina still exists, as a search on the database of the Linnaean collections (http://linnean-online.org/) yielded no results. Many incidents have happened which could have resulted in the loss of the original specimen used in 1758, from fires to trading away by other biologists to damage/degradation due to neglect (Gardiner & Morris, 2007). A new type specimen, MB07-033008 in Serra da Arrábida, Portugal is kept at as a holotype at the National Museum of Natural History and Science (Museu Nacional de História Natural e da Ciência or MUHNAC), University of Lisbon (Universidade de Lisboa) in Portugal when Wygodzinsky redescribed the species in 1941 (MUHNAC, 2014).
The species name, ‘saccharina’ literally means ‘starch eater’ (Linnaeus, 1758), in reference to its diet consisting primarily of starch and cellulose (Resh & Cardé, 2003).

Figure 16: The original article for the silverfish as described by Carl Linnaeus in 1758, in the 10th Edition of Systema Naturae. The genus and species names are boxed in red. As part of the earliest literature describing species, it contains little information and distinguishing features; Linnaeus started out with basic descriptions for the Genus in general (six legs, two palps, etc.), followed by a very brief Species description and its habitat/ distribution.
Synonyms
Lepisma argentina (Baker, 1780)
Lepisma quercetora (Wygodzinsky, 1945)
Lepisma vulgaris (Scopoli, 1763)
Tinea argentina (Baker, 1780)
Information provided by the Global Biodiversity Information Facility (Gbif) (Gbif.org, 2016).
Taxonavigation
This lineage of Lepisma saccharina is provided by UniProt Taxonomy.
› Eukaryota
-› Opisthokonta
--› Metazoa
---› Eumetazoa
----› Bilateria
-----› Protostomia
------› Ecdysozoa
-------› Panarthropoda
--------› Arthropoda
---------› Mandibulata
----------› Pancrustacea
-----------› Hexapoda
------------› Insecta
-------------› Dicondylia
--------------› Zygentoma (Thysanura)
---------------› Lepismatidae
----------------› Lepisma
Note that Zygentoma and Thysanura both appear in literature (see below).
Phylogeny
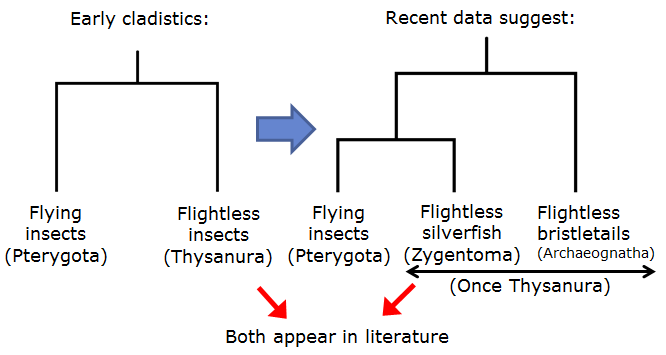
Figure 17: Illustration showing reclassification of Thysannura into two groups, Zygentoma and Archaeognatha.
Early cladistics classified bristletails with silverfish in the same Order, due to their flightless nature. Improvements in taxonomic knowledge, however, led to a revision of the name at the Order level (under classical Linnaean hierarchy) from Thysanura to Zygentoma to exclude jumping bristletails, now recognized as a different lineage in a separate Order, Archaeognatha (Mendes, 2002). As a result, one would notice that in older literature, they are referred as Thysanura (Which is currently recognized as a polyphyletic group) whereas in newer literature, both designations are used interchangeably when describing the silverfish taxa, although the new Zygentoma designation is favored.
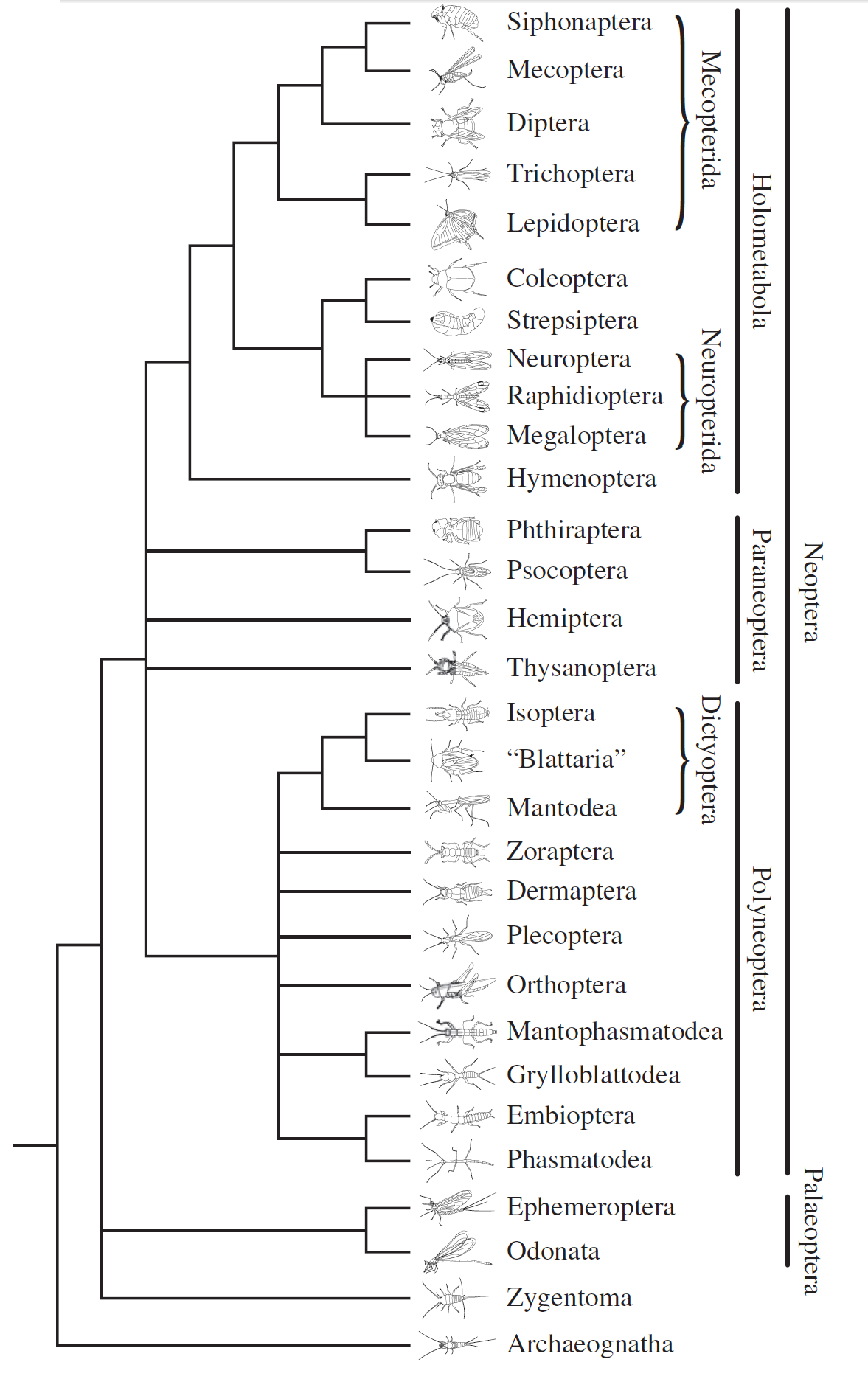
Figure 18: Reconstruction of relationships in Insects, based on catalytic subunit of DNA polymerase delta and the two largest subunits of RNA polymerase II from all the insect taxa sampled, constructed using inference by maximum likelihood and Bayesian analysis. Note that this technique in study is only capable of establishing that Thysanura is paraphyletic, and does not reflect timing of the appearance of each other taxa. Diagram from Ishiwata et al., 2011.
Ishiwata et al., 2011 was one such group contributing to the revision of taxas by showing that Thysanura was a polyphyletic clade. They constructed a diagram using representative members of major insect taxa by comparing the catalytic subunit of DNA polymerase delta and the two largest subunits of RNA polymerase in tracing ancestry. They obtained sufficient evidence that Archaeognatha (Brsitletails) is an outgroup to all other insects and Zygentoma (silverfishes) is an outgroup to winged insects (Pterygota, separated into Neoptera and Palecoptera in their study).
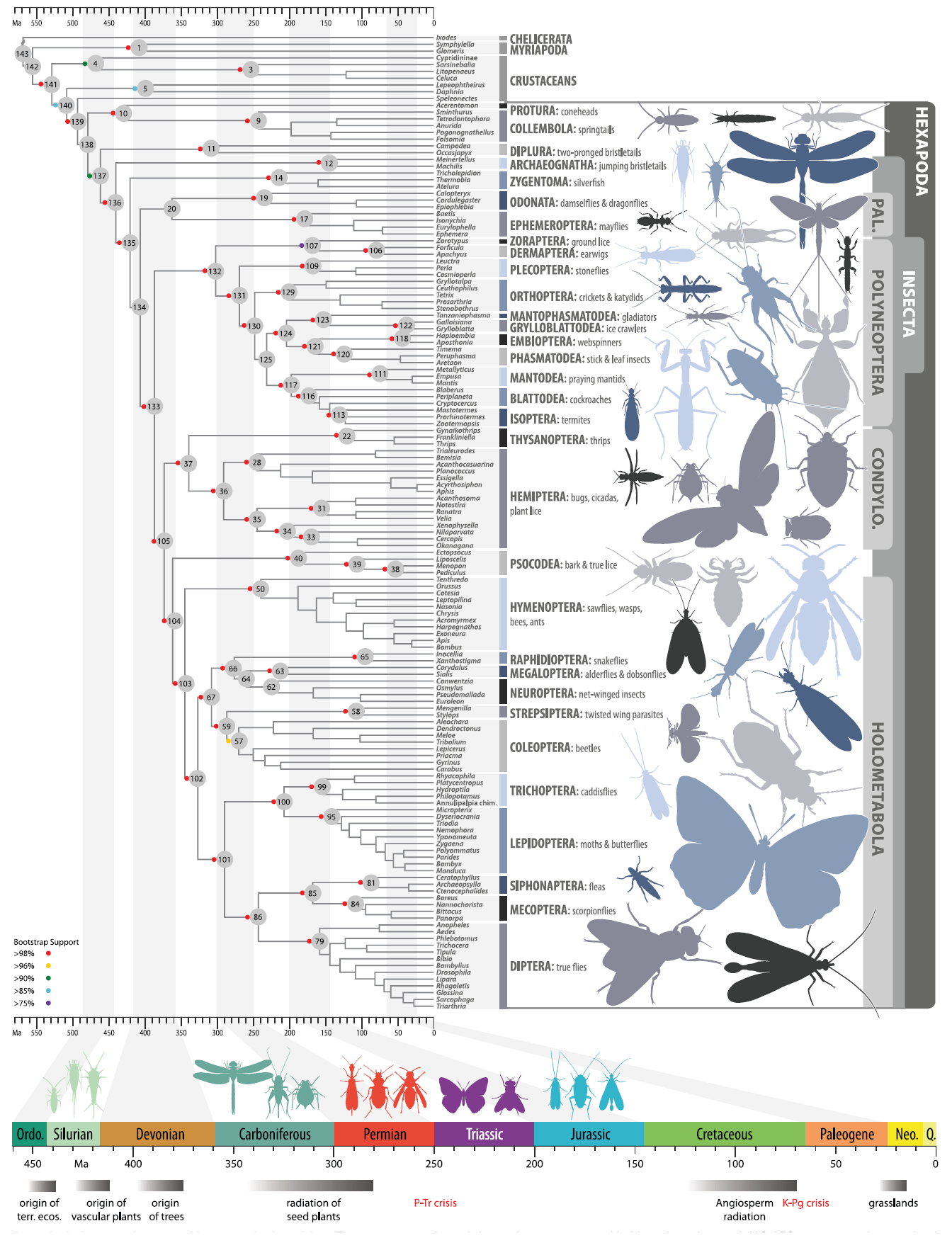
Figure 19: Full phylogenomics tree revealing the timing of appearance of each lineage in the insects, via a partitioned maximum likelihood analysis of amino acid sites. The tree was verified by Bootstrap analysis. Note that time of divergence is the best estimate based on a confidence interval, i.e. each time node is not a specific time set in stone, but the middle number in a range of probable time values. Diagram from Misof et al., 2014.
Misof et al., 2014 has revealed the timing of the appearance of the silverfish lineage to as early as during the Silurian period, though lack of fossil evidence leads to some uncertainty. The diagram revealed that hexapods (arthropods with six legs and three body segments) diverged from crustaceans; Insects then diverged in the early Ordovician, around the same time plants colonized land. The reason the silverfish is an insect, instead of only a hexapod, is due to presence of external mouthparts (mandibles), which other hexapods (e.g. springtails) lack.
More importantly, it confirmed an earlier divergence of bristletails from silverfish and flying insects (thus confirming results from Ishiwata et al., 2011 and reinforcing the decision to split Thysanura, since it wasn't monophyletic). It also revealed subsequent divergence of the silverfish lineage in the Early Devonian, where flightless silverfish diverged from flying insects. While modern silverfish are often considered to be 'primitive', they are still modern descendants. It just happens that its lineage retained more ancestral characters (most notably flightlessness and incomplete metamorphosis) compared to modern, flying insects.

Figure 20: A specimen of Tricholepidion gertschi (relict silverfish), for illustration and comparison purposes with other silverfish above. Photo credits: Samuel DeGrey on bugguide.net.
One common misconception is that the entire silverfish clade is a ‘living fossil’, representing an ancient clade of flightless insects. In truth, only the Lepidothrichidae taxa are generally considered as such, because the family contains two genera: Lepidotrix, which is extinct and known only from specimens preserved in Baltic amber, and an extant genus Tricholepidion, which contains a single living species, Tricholepidion gertschi resembling the fossil specimen (Engel, 2006). It is found, too, that Tricholepidion diverged from other siverfish in the late Triassic (~214 Mya), and it was found to be more closely related to silverfish than other flying insects, though it lost the head endoskeleton, styli, and coxal vesicles compared to most other extant silverfish (Misof et al., 2014). Silverfish are, nevertheless, still confirmed as a monophyletic group. To avoid confusion, it would be more appropriate to refer to extant silverfish as modern descendants retaining an ancestral trait (flightlessness), with a genus having one extant, modern species resembling extinct fossils.
Silverfish in Popular Culture
To end off with something lighthearted, ever wondered if silverfish are crawling around fictional worlds? While silverfish are often overlooked by popular culture, they do show up in a well-known game, Minecraft, as a cheeky insect which tends to appear out of nowhere in huge swarms, as a homage to its capability to appear out of cracks and crevices in homes. Thankfully, actual silverfish, as you recall from the very first video, are actually not persistent and dangerous, compared to their counterpart in popular gaming culture.
Figure 21: A silverfish, as represented in Minecraft. Personally I haven't played enough of the game to find out how notorious they really are, though they are definitely classified as in-game pests, sort of cute and actually slightly misleading representations of these harmless insects. Image credit: http://minecraft.gamepedia.com/Silverfish
Literature and References
Borror, D. J., De Long, D. M., & Triplehorn, C. A. (1981). An introduction to the study of insects. Saunders College Publishing. New York. NY, 10017.
Delany, M. J. (1954). Thysanura and Diplura. Handbooks for the Identification of British Insects, Vol. 1, No. 2. Royal Entomological Society, London. Pp. 1-7.
DeVries, Z. C., & Appel, A. G. (2013). Standard metabolic rates of Lepisma saccharina and Thermobia domestica: Effects of temperature and mass. Journal of insect physiology, 59(6), 638-645.
Encyclopedia of Life,. (2016). Bristletail - Lepisma saccharina - Details. Encyclopedia of Life. Accessed via http://www.eol.org/pages/1022755/details#habitat on 2016-11-05.
Engel, M. S. (2006). A note on the relic silverfish Tricholepidion gertschi (Zygentoma). Transactions of the Kansas Academy of Science, 109(3), 236-238.
Evans, J. D., & Gundersen-Rindal, D. (2003). Beenomes to Bombyx: future directions in applied insect genomics. Genome biology, 4(3), 1.
Gallo, F. (1961). Biological agents which damage paper materials in libraries and archives. Studies in Conservation, 6(sup1), 55-61.
Gardiner, B. & Morris, M. (2007). The Linnaean collections. Oxford: Wiley-Blackwell.
Gbif.org,. (2016). Lepisma saccharina Linnaeus - Checklist View. Gbif.org. Accessed via http://www.gbif.org/species/107607285 on 2016-11-05.
Grimaldi, D. & Engel, M. (2005). Evolution of the insects. Cambridge [U.K.]: Cambridge University Press.
Hasenfuss, I. V. A. R. (2002). A possible evolutionary pathway to insect flight starting from lepismatid organization. Journal of Zoological Systematics and Evolutionary Research, 40(2), 65-81.
Hasenfuss, I. V. A. R. (2008). The evolutionary pathway to insect flight: a tentative reconstruction. Arthropod Syst. Phylogeny, 66, 19-35.
Houseman, R. (2016). G7376 Silverfish and Firebrats | University of Missouri Extension. Extension.missouri.edu. Accessed via http://extension.missouri.edu/publications/DisplayPrinterFriendlyPub.aspx?P=g7376 on 2016-11-22.
Ishiwata, K., Sasaki, G., Ogawa, J., Miyata, T., & Su, Z. H. (2011). Phylogenetic relationships among insect orders based on three nuclear protein-coding gene sequences. Molecular Phylogenetics and Evolution, 58(2), 169-180.
Klass, C. (2009). Silverfish and Firebrats. Dept. of Entomology, Cornell University, Ithaca NY.
Koehler, P., Branscome, D., Oi, F., & Bayer, B. (2016). Booklice and Silverfish. Edis.ifas.ufl.edu. Accessed via http://edis.ifas.ufl.edu/ig094 on 2016-11-05.
Koehler, P., Buss, E., Kern, W., Pereira, R., & Baldwin, R. (2016). Pests in and around the southern home. Florida.
Linnaeus, C. (1758). Systema Naturae. 1 (10th ed.). Pp. 608.
Lindsay, E. (1940). The biology of the silverfish, Ctenolepisma longicaudata Esch. with particular reference to its feeding habits. Proceedings of the Royal Society of Victoria, 52(pt. 1), 35-83.
Mendes, L. F. (2002). Taxonomy of Zygentoma and Microcoryphia: historical overview, present status and goals for the new millennium: Proceedings of the Xth international Colloquium on Apterygota, České Budějovice 2000: Apterygota at the Beginning of the Third Millennium. Pedobiologia, 46(3), 225-233.
Misof, B., Liu, S., Meusemann, K., Peters, R. S., Donath, A., Mayer, C., ... & Niehuis, O. (2014). Phylogenomics resolves the timing and pattern of insect evolution. Science, 346(6210), 763-767.
Mittmann, B., & Scholtz, G. (2001). Distal-less expression in embryos of Limulus polyphemus (Chelicerata, Xiphosura) and Lepisma saccharina (Insecta, Zygentoma) suggests a role in the development of mechanoreceptors, chemoreceptors, and the CNS. Development genes and evolution, 211(5), 232-243.
Morita, H. (1926). Some observations on the “silverfish”. Lepisma saccarina L.
National Museum of Natural History and Science, University of Lisbon. (2014). Insect Collection from the Museu Nacional de História Natural e da Ciência, Universidade de Lisboa, Portugal. Accessed via http://www.gbif.org/occurrence/1022889857 on 2016-11-05.
Resh, V. & Cardé, R. (2003). Encyclopedia of insects. Amsterdam: Academic Press. Pp. 1204.
Rost-Roszkowska, M. M., Piłka, M., Szymska, R., & Klag, J. (2007). Ultrastructural studies of midgut epithelium formation in Lepisma saccharina L.(Insecta, Zygentoma). Journal of morphology, 268(3), 224-231.
Sahoo, J. (1990). Preservation of library materials: Some preventive measures. OHRJ, 47(1), 105-114.
Schaller, F. (1968). Soil Animals. University of Michigan Press, Ann Arbor, Mich. Pp. 144.
Sturm, H. (1956). Die Paarung beim Silberfischchen Lepisma saccharina. Zeitschrift für Tierpsychologie, 13(1), 1-12.
Sun, J.S., & Scharf, M. (2010). Exploring and integrating cellulolytic systems of insectsto advance biofuel technology. Insect Science. 17(3), 163-165.
Tree of Life Web Project. (1995). The Tree of Life Web Project, Version 01 January 1995 (temporary). Accessed via http://tolweb.org/ on 2016-11-02.
Wang, S. Y., Lai, W. C., Chu, F. H., Lin, C. T., Shen, S. Y., & Chang, S. T. (2006). Essential oil from the leaves of Cryptomeria japonica acts as a silverfish (Lepisma saccharina) repellent and insecticide. Journal of Wood Science, 52(6), 522-526.
Wygodzinsky, P. W. (1972). A review of the silverfish (Lepismatidae, Thysanura) of the United States and the Caribbean area. American Museum novitates; no. 2481.